News Releases
SUNNYVALE, Calif., June 9, 2020 /PRNewswire/ -- Cepheid today announced the development of a next-generation test to assist global efforts in the fight against the spread of COVID-19 during the upcoming respiratory virus season. The Xpert® Xpress SARS-CoV-2/Flu/RSV four-in-one test is expected to deliver qualitative detection of SARS-CoV-2, Flu A, Flu B and RSV from a single patient sample. The test is designed for use on any of Cepheid's more than 25,000 GeneXpert® Systems placed worldwide, with results expected in as little as 35 minutes.
"Patients infected by SARS-CoV-2, Flu A, Flu B, and RSV have overlapping clinical presentations, but fundamentally different treatment and management pathways," said Dr. David Persing, MD, Ph.D., Chief Medical and Technology Officer at Cepheid. "Unlike the common cold viruses, infection with these four viruses is often associated with fever and other systemic manifestations that may be coupled with severe outcomes, especially in the elderly."
In the coming weeks, Cepheid intends to pursue the FDA's Emergency Use Authorization (EUA) pathway for regulatory authorization and make the test available globally on its cartridge-based GeneXpert Systems, which features instruments that can be configured for both near patient point-of-care and high volume laboratory testing needs.
"On March 20th, Cepheid was granted the first-ever EUA by the FDA for use in point-of-care settings for our Xpert Xpress SARS-CoV-2 test," said Cepheid President Warren Kocmond. "Since then, we have experienced unprecedented demand for this technology. Leveraging the quality design of Xpert Xpress SARS-CoV-2 and our widely utilized Xpert Xpress Flu/RSV tests, we're combining two world-class products in a single, rapid solution ahead of the upcoming flu season. This will enable our customers to have increased testing throughput on their current GeneXpert System and increase our ability to provide supply continuity for the market."
Visit www.cepheid.com/coronavirus for latest updates on our SARS-CoV-2 tests.
GeneXpert's Modular Design Enables High-Volume Laboratory and Near-Patient Point-of-Care Testing
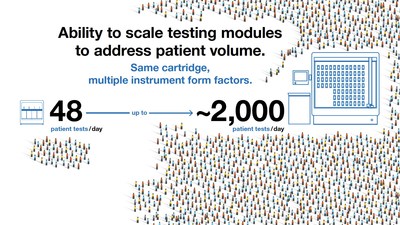
The GeneXpert System was built for simple, reference lab quality PCR testing – on location at medical centers and hospitals or closer to patient in health clinics and nursing homes. At the core of every GeneXpert System is the module (or testing bay) where a test cartridge is loaded onto the machine. Our line of GeneXpert Systems can be configured with a varying number of modules, or test bays, to meet the volume requirements of any setting. Smaller GeneXpert Systems are configured with 2 or 4 modules – meaning up to four different tests can be run at one time. Our largest GeneXpert System is configured with up to 80 modules – meaning as many as 80 tests can operate independently at any given time with a capacity of about 2,000 tests per day*.
About Cepheid
Based in Sunnyvale, Calif., Cepheid is a leading molecular diagnostics company that is an operating company within Danaher Corporation's (NYSE: DHR) Diagnostics platform. Cepheid is dedicated to improving healthcare by developing, manufacturing, and marketing accurate yet easy-to-use molecular systems and tests. By automating highly complex and time-consuming manual procedures, the company's solutions deliver a better way for institutions of any size to perform sophisticated molecular diagnostic testing for organisms and genetic-based diseases. Through its strong molecular biology capabilities, the company is focusing on those applications where accurate, rapid, and actionable test results are needed most, such as managing infectious diseases and cancer. For more information, visit http://www.cepheid.com.
*Cepheid internal study based on 30 minute test results.
For Cepheid Media Inquiries:
Darwa Peterson
darwa.peterson@cepheid.com
Media Contacts:
Jason Spark,
CanaleComm, jason@canalecomm.com
Cammy Duong,
CanaleComm, cammy@canalecomm.com
SOURCE Cepheid